Bloodborne Pathogens and Needlestick Prevention
Evaluating and Controlling Exposure
Engineering Controls
Engineering controls are defined in OSHA's Bloodborne Pathogen standard as controls that isolate or remove the bloodborne pathogen hazard from the workplace [29 CFR 1910.1030(b)]. The standard states that "engineering and work practice controls shall be used to eliminate or minimize employee exposure" [29 CFR 1910.1030(d)(2)(i)]. This means that if an effective and clinically appropriate control, such as a safety-engineered sharp exists, an employer must evaluate and implement it.
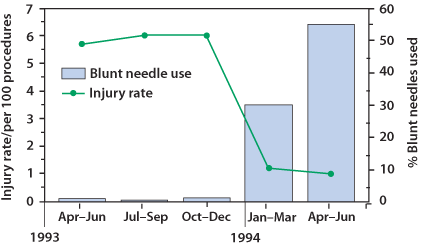
Studies have shown that as many as one-third of all sharps injuries in the hospital setting occur during sharps disposal. Nurses are particularly at risk, as they sustain the greatest number of needlestick injuries. The Centers for Disease Control and Prevention (CDC) estimates that 62 to 88 percent of sharps injuries can be prevented simply by using safer medical devices such as blunt suture needles (Figure 3). The following references provide information regarding possible solutions for bloodborne pathogens and needlestick hazards.
Control Programs
- A Best Practices Approach for Reducing Bloodborne Pathogen Exposures. Describes engineering and work practice control improvements (2001).
- Model Plans and Programs for the OSHA Bloodborne Pathogens and Hazard Communications Standards. OSHA Publication 3186, (2003). Includes a model exposure control plan that meets the requirements of the OSHA Bloodborne Pathogens Standard and can be tailored to meet the specific requirements for an establishment.
- Preventing Exposures to Bloodborne Pathogens among Paramedics. U.S. Department of Health and Human Services (DHHS), National Institute for Occupational Safety and Health (NIOSH) Publication No. 2010-113, (April 2010).
- Bloodborne Pathogens - Personal Protective Equipment (PPE) Reduces Exposure to Bloodborne Pathogens. OSHA Fact Sheet, (January 2011).
- Bloodborne Pathogens - Hepatitis B Vaccination Protection. OSHA Fact Sheet, (January 2011).
- Information for Employers Complying with OSHA's Bloodborne Pathogens Standard. U.S.
- HIV and Occupational Exposure. Centers for Disease Control and Prevention (CDC). Offers recommendations to prevent transmission of HIV to healthcare personnel in the workplace (2013).
- Stop Sticks Campaign: Devices with Sharps Injury Protection Features. National Occupational Research Agenda (NORA) offers links to organizations that provide information on evaluating sharps devices and lists of safer devices (2019).
- A Best Practices Approach for Reducing Bloodborne Pathogen Exposures. Cal/OSHA Consultation Service, Department of Industrial Relations, (2001).
- Safety in surgery:
- American College of Surgeons. Revised Statement on Sharps Safety. (2016).
- Association of Perioperative Registered Nurses. AORN Guidance Statement: Sharps Injury Prevention in the Perioperative Setting. (2005).
- International Safety Center provides the Exposure Prevention Information Network (EPINet®) free of charge to healthcare facilities around the world as a means to standardize methods for recording and tracking needlesticks, sharps injuries, and blood/body fluid exposure incidents. EPINet consists of "Reports for both Needlestick and Sharp Object Injuries" and "Blood and Body Fluid Exposures". EPINet publishes very detailed data from its surveillance network annually. Data includes information required by the Sharps Injury Log as well as additional information about each exposure incident that allows facilities to assess whether they have the appropriate controls and protections in place.
- eTool: Hospitals - Hospital-wide Hazards - Biological Hazards – Infectious Diseases. Occupational Safety and Health Administration. This discusses preventing transmission of bloodborne and of other infectious agents spread by contact, droplet, and the airborne route.
Safer Needle Devices
- Needlestick and Other Risks from Hypodermic Needles on Secondary I.V. Administration Sets - Piggyback and Intermittent I.V. Food and Drug Administration (FDA) Safety Alert, (April 16, 1992). Urges the use of needleless systems or recessed needle systems to reduce the risk of needlestick injuries.
- Evaluation of Blunt Suture Needles in Preventing Percutaneous Injuries Among Health-Care Workers During Gynecologic Surgical Procedures; New York City, March 1993-June 1994. Centers for Disease Control and Prevention (CDC), Morbidity and Mortality Weekly Report (MMWR) 46(02);25-29, (January 17, 1997). Identifies the effectiveness of blunt needles in reducing percutaneous injuries (PIs) and suggests that they should be considered for more widespread use in surgical procedures.
- Evaluation of Safety Devices for Preventing Percutaneous Injuries Among Health-Care Workers During Phlebotomy Procedures -- Minneapolis-St. Paul, New York City, and San Francisco, 1993-1995. Centers for Disease Control and Prevention (CDC), Morbidity and Mortality Weekly Report (MMWR) 46(02);21-25, (January 17, 1997). Indicates that the use of phlebotomy safety devices significantly reduces phlebotomy-related percutaneous injury (PI) rates.
- Lessons Learned in Safety-Device Implementation (2007).
- Use of Blunt-Tip Suture Needles to Decrease Percutaneous Injuries to Surgical Personnel: Safety and Health Information Bulletin. OSHA and the National Institute for Occupational Safety and Health (NIOSH) Publication No. 2008-101, (October 2007). Supersedes NIOSH Publication 2007-132.
- Workbook for Designing, Implementing, and Evaluating a Sharps Injury Prevention Program. Centers for Disease Control and Prevention (CDC).
- FDA, NIOSH & OSHA joint safety communication: Blunt-tip surgical suture needles reduce needlestick injuries and the risk of subsequent bloodborne pathogen transmission to surgical personnel. CDC (2012).
Decontamination
Selected EPA-registered Disinfectants and FDA-Cleared Sterilants and High-Level Disinfectants.
- EPA's Registered Antimicrobial Products Effective Against Bloodborne Pathogens: Human immunodeficiency virus (HIV), Hepatitis B and Hepatitis C [List S] (2024)
- List A: Antimicrobial Products Registered with the EPA as Sterilizers. US EPA (2024).
- FDA-Cleared Sterilants and High-Level Disinfectants with General Claims for Processing Reusable Medical and Dental Devices FDA (2023).
Post-exposure Evaluation
According to EPINet® data, the 2021 average daily census for needlestick and sharp object injuries was 31 needlesticks per 100, based on 41 hospitals reporting. Percutaneous injuries (PIs) may be caused by sharp objects such as hypodermic needles, scalpels, suture needles, wires, trochanters, or surgical pins. Additionally, PIs may also be caused by saws and sharp objects deliberately contaminated with blood or body fluids used to inflict harm on law enforcement personnel. Other exposure incidents include splashes and other contact with mucous membranes or non-intact skin. Post-exposure management is an integral part of a complete program for preventing infection following exposure incidents and is to be conducted by or under the supervision of a licensed physician or other licensed healthcare professional. The following references provide useful information about the management of occupational exposure incidents to blood or other potentially infectious materials.
- A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States. Centers for Disease Control and Prevention (CDC), Morbidity and Mortality Weekly Report (MMWR) 55(RR16);1-25, (December 8, 2006).
- Immunization of Health-Care Personnel (cdc.gov) Recommendations of the Advisory Council on Immunization Practices (ACIP)
- Bloodborne Pathogens - Bloodborne Pathogen Exposure Incidents. OSHA Fact Sheet, (January 2011).
- Recommendations of the Advisory Committee on Immunization Practices for Use of a Hepatitis B Vaccine with a Novel Adjuvant | MMWR. CDC. In 2018, the CDC updated guidance to include acceptance of a two-dose Hepatitis B vaccine as recommended by the Advisory Committee on Immunization Practices (ACIP).
- Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (2018).
- Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. (September 25, 2023; Updated May 23, 2018).
- HIV Testing. Centers for Disease Control and Prevention (CDC). This page discusses HIV testing, new testing techniques as well as provides information regarding rapid testing and how the tests can be implemented in different settings and research on the effectiveness and possible uses of the tests (2022).
- PEP: Post Exposure Prophylaxis. The National HIV/AIDS Clinician's Consultation Center (2024).

