Ionizing Radiation
Background
What is Ionizing Radiation?
Atoms are the basic building blocks of all matter, and atoms of different elements occur naturally on the earth. Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in orbitals around the nucleus. The number of protons determines the element (oxygen, hydrogen, etc.), and the number of neutrons determines the isotope (hydrogen-1, hydrogen-2, etc.). Some isotopes are unstable and give off energy (i.e., decay) to become more stable. In this context, an unstable atom is said to be "radioactive," and the energy it releases is referred to as "radiation." When the radiation has enough energy to ionize other atoms (i.e., remove negatively-charged particles called "electrons") in its path, it is referred to as "ionizing radiation." Types of ionizing radiation include alpha, beta, and neutron particles; gamma rays; and X-rays. In contrast, non-ionizing radiation has a lower energy that is not capable of ionizing other atoms. Examples include radio waves, microwaves, and visible light.
For more information about the types of ionizing radiation, see the What are the types of ionizing radiation? section.
Radionuclides (also known as radioisotopes) are elements in an unstable form, which are radioactive but will lose their radioactivity over time through radioactive decay as they change to a more stable isotope or element. Each radionuclide decays at a different rate.
What are the Sources of Ionizing Radiation?
Sources of ionizing radiation include radioactive materials and radiation-generating machines.
Radioactive materials can be naturally occurring (such as uranium and radium found in the earth) or manmade in an accelerator or reactor.
Radiation-generating machines, such as medical X-ray machines, produce ionizing radiation electronically and stop producing radiation when turned off. Equipment that contains radioactive material, such as some industrial radiography equipment, cannot be turned off because the radioactive source emits ionizing radiation. These sources must be shielded (i.e., surrounded by a material that can block radiation) to prevent or reduce radiation exposure.
What are the Types of Ionizing Radiation?
Five types of ionizing radiation—alpha particles, beta particles, positrons, gamma rays, and X-rays—are the primary focus of this Ionizing Radiation Safety and Health Topics page.
This page also introduces another type of ionizing radiation, neutron particles, although significant worker doses from neutrons are most likely near reactors or when using neutron sources (e.g., californium (Cf)-252, americium (Am)-241/beryllium (Be), plutonium (Pu) Be). Worker doses from neutrons could also occur in certain radiological emergencies. Visit OSHA’s Radiation Emergency Preparedness and Response page for more information on protecting workers during radiological emergencies.
| Type of ionizing radiation | Examples |
|---|---|
| PARTICULATE RADIATION (sub-atomic particles with mass, such as alpha and beta particles, electrons, and neutrons) | |
|
Alpha particles (α) Positively charged particles consisting of two protons and two neutrons emitted from the nucleus of some radioactive atoms. An alpha particle is the nucleus of a helium atom. Unstable atoms with a low neutron-to-proton ratio may emit alpha particles. |
Radionuclides that emit alpha particles include:
For example, a Po-210 atom has 84 protons and 126 neutrons, and is unstable (i.e., radioactive). To become more stable, the Po-210 atom ejects an alpha particle, consisting of two protons and two neutrons. Having lost two protons and two neutrons, the radioactive Po-210 atom becomes stable lead-206 (Pb-206), with 82 protons and 124 neutrons. |
|
Beta particles (β-) and Positrons (β+) Beta particles (β-) Negatively-charged, fast-moving electrons emitted from the nucleus of various radionuclides. Unstable atoms with a high neutron-to-proton ratio emit negatively-charged beta particles. Positrons (β+) Positively-charged, fast-moving electrons emitted from the nucleus of certain radionuclides. Unstable atoms with a low neutron-to-proton ratio can emit positrons. |
Beta particles (β-) Some radionuclides that emit beta particles include:
For example, a carbon-14 atom has six protons and eight neutrons, and is unstable (i.e., radioactive). To become more stable, the C-14 atom releases radiation by turning a neutron into a proton and ejecting an electron (i.e., a beta particle). Having gained a proton and lost a neutron, the radioactive C-14 atom becomes stable nitrogen-14 (N-14), with seven protons and 7 neutrons. Positrons (β+) Fluorine-18 (F-18) is an example of a positron-emitting radionuclide that is commonly used in medical facilities for positron emission tomography (PET) scanning. An F-18 atom has nine protons and nine neutrons, and is unstable (i.e., radioactive). To become more stable, the F-18 atom releases radiation by turning a proton into a neutron and ejecting a positron. Having gained a neutron and lost a proton, the radioactive F-18 atom becomes stable oxygen-18 (O-18), with eight protons and 10 neutrons. |
|
Neutron particles Neutral (i.e., having no electric charge) particles that can be emitted from the nuclei of various unstable radionuclides. Neutrons are high-speed nuclear particles that are the only type of ionizing radiation that can make objects radioactive. |
Nuclear fission and fusion reactions, as well as neutron sources (e.g., Cf-252, AmBe), neutron generators, and some particle accelerators, produce neutrons. For example, neutrons would be produced from the detonation of a fissile nuclear weapon, such as an improvised nuclear device (IND). Visit OSHA’s Radiation Emergency Preparedness and Response page for more information. |
| ELECTROMAGNETIC RADIATION (Gamma rays and X-rays) has no mass and no charge. | |
|
Gamma rays (γ) High-energy electromagnetic photons emitted from the nucleus of an unstable, excited atom. Gamma rays are pure energy and can travel great distances at high speed. |
Some radionuclides that emit gamma rays include:
Gamma rays are often emitted along with alpha or beta particles during radioactive decay (e.g., Co-60, Ir-192). |
|
X-rays High-energy electromagnetic photons emitted from outside the nucleus. The primary difference between X-rays and gamma rays is that X-rays are emitted from processes outside the nucleus, but gamma rays originate inside the nucleus. |
Some radionuclides that emit X-rays include:
Machines containing an X-ray tube also electronically produce X-rays. |
What Determines the Amount of Radioactive Decay or Radiation Emitted?
Radioactive decay is a process by which unstable (i.e., radioactive) atoms release energy (i.e., radiation) to become more stable.
The rate of radioactive decay for each radioactive element is described by its half-life, or the amount of time it takes for around half of the radioactive atoms present to decay to a more stable form. Half-lives for different radioactive elements vary from fractions of seconds to billions of years.
Unstable radioactive atoms can go through a series of decays, or disintegrations, before reaching a stable form. For example, uranium-238 (U-238), which occurs naturally in Earth’s crust, has a half-life of 4.5 billion years and decays by alpha particle emission to thorium-234 (Th-234). Th-234 has a half-life of 24 days and decays by beta particle emission to protactinium-234m (Pa-234m), which has a half-life of only 1.2 minutes and decays by beta particle emission to uranium-234 (U-234, half-life of 240,000 years). As shown in the aturally-occurring U-238 decay series, the final product of the decay series is lead-206 (Pb-206), which is stable. Of note in this series is radon-222 (Rn-222), a radioactive gas that poses an inhalation hazard to workers and the public (see the Hazard Recognition page).

Source: U.S. Geological Survey
Uranium-238 decay series (naturally-occurring), which produces alpha, beta, and gamma radiation (not shown).
How is Radioactivity Described and Measured?
Radioactivity is the number of energized particles or photons emitted by a source of radioactive material per unit of time. Another way to describe radioactivity is the number of decays (also described as disintegrations) occurring per unit of time.
Units of measurement for radioactivity are the Curie (Ci, traditional U.S. unit) or Becquerel (Bq, international unit).
- 1 Bq = 1 decay per second
- 1 Ci = 3.7x1010 Bq
What Happens to the Radiation Emitted?
Ionizing radiation particles (e.g., alpha, beta) or high-energy photons (gamma rays, X-rays) can travel different distances and interact with the atoms of absorbing materials in their paths, causing excitation or ionization of the atoms. As shown in the graphic and table below, while alpha and beta particles are not very penetrating through other materials, gamma and X-rays are quite penetrating, as are neutrons.

Source: OpenClipArt
The illustration shows the penetrating power of different types of ionizing radiation, ranging from the least penetrating alpha particles to the most penetrating neutrons.
When ionizing radiation interacts with humans, it is capable of damaging living cells in the human body. Humans can be exposed: 1) to external radiation from a radiation source outside of the body, such as an X-ray from an X-ray machine; or 2) through internal exposure following inhalation (breathing in), ingestion (swallowing), or wound uptake (i.e., through non-intact skin) of radioactive material. In addition, the skin can become contaminated with radioactive materials when proper controls are not in place to prevent contamination or following an emergency.
| Type of ionizing radiation | How it travels and penetrates | How it delivers dose to the body |
|---|---|---|
| Alpha particles (α) | Alpha particles cannot penetrate most other materials. A piece of paper, the dead outer layers of skin, or even a few inches of air are sufficient to stop alpha particles. | Radioactive material that emits alpha particles can be very harmful to living cells when alpha particles are inhaled, ingested, or absorbed into the blood stream (e.g., through a cut in or area of non-intact skin). |
| Beta particles (β) | Beta particles can travel up to several feet in the air. Beta particles can be stopped by some plastics, aluminum, or a block of wood. Beta particles should never be shielded with lead or other high atomic number shields, which could result in X-rays being released. | Some beta particles are capable of penetrating the skin and causing radiation damage, such as skin burns. Beta particles are most harmful to living cells when they are inhaled or ingested. |
| Gamma rays (γ) and X-rays (electromagnetic radiation) | Gamma rays and X-rays are very penetrating and can travel great distances. Lead or concrete is able to reduce the intensity of gamma rays and X-rays. | Gamma rays and X-rays can easily pass completely through the human body; however, a fraction of the energy can be absorbed by tissue and can damage living cells. |
| Neutron particles | Neutrons have an exceptional ability to penetrate materials. Hydrogen-containing materials (concrete or water) are best for shielding neutrons. | Neutrons can contribute significantly to radiation dose. |
More information about the health effects of ionizing radiation is provided on the Health Effects page.
What is Radiation Exposure?
Exposure. is defined as the amount of X-ray or gamma radiation that interacts in a volume of air.
Units of measurement for radiation exposure are the Roentgen (R, traditional U.S. unit) or Coulomb/kilogram (C/kg, international unit).
The Hazard Recognition page provides information about external exposure and internal exposure.
What is Radiation Dose?
Absorbed dose is the amount of radiation energy absorbed per unit mass (i.e., an individual, tissue, or material).
Units of measurement for absorbed dose are the rad (traditional U.S. unit) or Gray (Gy, international unit).
- 1 Gy = 1 J/kg = 100 rad
Equivalent dose is the amount of radiation absorbed by an individual (i.e., the absorbed dose), multiplied by a weighting factor (WR) that adjusts for the damaging potential of the type of radiation.
Units of measurement for equivalent dose are the rem (traditional U.S. unit) or Sievert (Sv, international unit). Relevant equivalencies include:
- 1 Sv = 100 rem
- Equivalent dose (rem) = Absorbed dose (rad) * WR
- Equivalent dose (Sv) = Absorbed dose (Gy) * WR
- For beta, X-ray, and gamma radiation, WR = 1, therefore the equivalent dose is equal to the absorbed dose (i.e., 1 rad absorbed dose = 1 rem equivalent dose).
- For alpha particles, WR = 20 (i.e., 1 rad absorbed dose = 20 rem equivalent dose).
- For neutrons, WR varies based on the energy. The equivalent dose is higher per unit of absorbed dose due to the damaging potential of neutron radiation.
Effective dose is the dose to the whole body that carries with it the same risk as a higher dose to a portion of the body.1 Tissue weighting factors are assigned to various body parts, based upon their sensitivity to ionizing radiation (i.e., radiosensitivity), for the risks of lethal cancer and serious prompt genetic effects. The effective dose is the sum of the tissue-weighted equivalent doses.
For example, suppose the lungs received an equivalent dose of 8 rem (0.08 Sv). Using the weighting factor of 0.12 recommended for the lungs by the International Commission on Radiological Protection (ICRP)2 and assuming no other body parts were exposed, the effective dose would be: 8 rem (0.08 Sv) * 0.12 = 1 rem (0.01 Sv). Therefore, in this example, an equivalent dose of 8 rem (0.08 Sv) to the lungs carries with it the same risk as a dose of 1 rem (0.01 Sv) to the whole body.
Units for effective dose are also the rem (traditional U.S. unit) or Sievert (Sv, international unit).
- 1 Sv = 100 rem
Note that OSHA’s Ionizing Radiation standards (including 29 CFR 1910.1096 in general industry and, to the extent it applies, shipyard employment, marine terminals, and longshoring; and 29 CFR 1926.53 in construction) are based on limiting the dose to the most critically exposed part of the body, such that they do not use effective dose. See the definition of “dose” in paragraph (a)(5) of the general industry standard (29 CFR 1910.1096), which reflects the quantity of ionizing radiation absorbed, per unit of mass, by the body or by any portion of the body.
The National Council on Radiation Protection and Measurements (NCRP) estimated in 2009 that the annual average radiation dose per person in the United States from natural background radiation and medical exposures is about 620 mrem (6.2 mSv). About half of the dose comes from natural sources and the other half from medical exposures. The chart details NCRP’s estimates of the radiation sources contributing to this total annual dose.
Sources of ionizing radiation for estimated annual average radiation dose per person in the U.S.
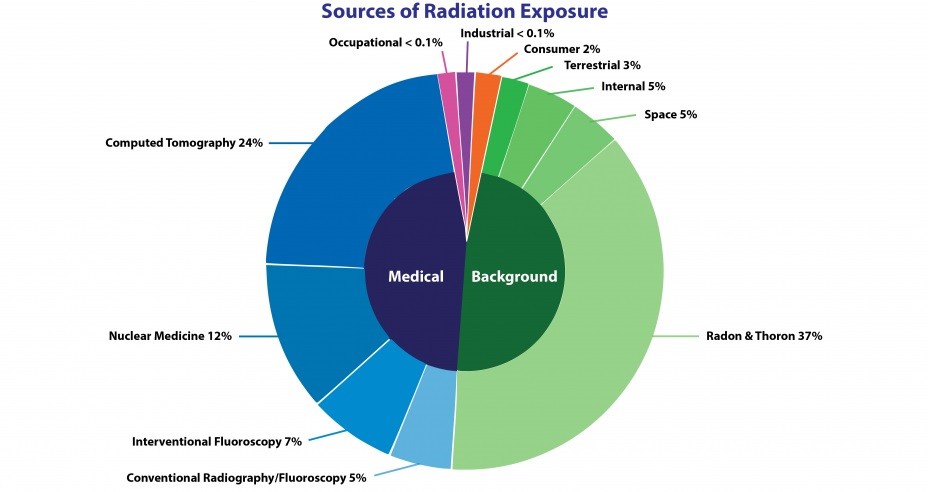
According to NCRP, the annual average radiation dose per person in the United States from natural background radiation and medical exposures is about 620 mrem (6.2 mSv).
Terminology used in figure:
- Computed tomography (CT): A medical imaging procedure that uses X-rays to show cross-sectional images of the body.
- Interventional fluoroscopy: The use of ionizing radiation to guide small instruments such as catheters through blood vessels or other pathways in the body.
- Conventional radiography and fluoroscopy: Radiography is the use of X-ray machines by doctors and dentists to view the human body. Fluoroscopy is a medical technique used by doctors to take real-time moving images of internal structures in the body by placing a patient between a fluorescent screen and an radiography and X-ray source.
- Nuclear medicine: Radioactive elements or tracers that are given intravenously or orally. A gamma camera detects gamma rays emitted by the tracer. These data are fed into a computer where they are used to produce images and other information about the body’s organ system.
Adapted from: Report No. 160: Ionizing Radiation Exposure of the Population of the United States, National Council on Radiation Protection and Measurements (NCRP); and Radiation Education Activities, U.S. Environmental Protection Agency (EPA).
Radiation dose can be grouped into two categories based on the duration (length of time period) of exposure to the radiation source:
- Acute dose occurs in a matter of minutes, hours, or a few days.
- Chronic dose is continuous or intermittent exposure to radiation occurring over a long period of time.
What are Radiation Caution Symbols?
All workers should know how to recognize radiation caution or warning symbols, which are posted to alert people about the health hazards of radiation sources.
The international, three-cornered symbol (trefoil) of radiation can be magenta, purple, or black, on a yellow background.
Another international radiation warning symbol with radiating waves, a skull and crossbones, and a running person, can be used as a supplemental symbol for dangerous (i.e., life threatening), high-activity radiation sources.
Some higher hazard industrial radiography equipment containing shielding also features the trefoil symbol on the outside. The inside of such equipment features the supplemental dangerous radiation warning symbol to provide an additional warning about the dangers of the much greater radiation exposure possible from the unshielded radioactive material inside that equipment. This additional symbol warns workers not to disassemble the equipment or otherwise disrupt the shielding in place.
1 Health Physics Society, Doses from Medical X‐Ray Procedures.
2 International Commission on Radiological Protection (ICRP), Publication No. 103, "The 2007 Recommendations of the ICRP."





